Chemical composition of honey

Bees are adapted to highly specialized food. From nectar and pollen, they receive all the substances they need to reproduce, grow, develop and perform numerous works.
Honey is a product of bee processing of plant nectar. It is a very sweet, viscous aromatic liquid with a peculiar taste and smell, a variety of colors – from clear, light or slightly yellow to bright yellow, brown, dark brown and dark. The color of honey mainly depends on the type of plants, the nectar of which is collected and processed by bees.
The totality of processes for processing nectar in the nest of bees is called the Ripening of honey . Mature honey is honey packed in cells and sealed with waxen (impermeable) lids; wax seal of honey is an indicator of completeness of biochemical processes that convert nectar into honey.
Honey, pumped out on a honey extractor (and sometimes in honeycombs), crystallizes, that is, loses its color and transparency, turning into a homogeneous pulp-like mass, or even acquires a solid structure. The taste and nutritional properties of honey are fully preserved. But for bees, the crystallization of honey in the cells is dangerous: crystallized honey honey bees can not be pulled through the proboscis and used for feeding in winter and early spring.
The composition of honey is largely determined by the content of nectar, which the bees collected and processed in the hive.
The main mass of honey is dissolved in water sugar – glucose (grape sugar) and fructose (fruit sugar). The solubility in water of these sugars is from 16 to 22% (on average 19%) of honey mass. If you exclude water, then sugar accounts for about 95% of all dry matter. The share of other substances, and they number in honey over 50, account for about 5%.
Glucose and fructose are carbohydrates belonging to the group of monosaccharides. These are the simplest in the chemical structure of
Glucose constitutes 31-38% of honey sugars. It crystallizes faster than other sugars. Fructose accounts for 38-43% of all honey sugars. Fructose, unlike glucose, slowly crystallizes and is more hygroscopic than other sugars. The composition of honey includes small amounts of maltose, raffinose and melibose.
In addition to glucose and fructose, honey contains up to 2% sucrose (cane, sugar beet). This sugar belongs to the group of disaccharides; it under the influence of the enzyme invertase decomposes into equal parts of glucose and fructose. Sucrose in honey is the remains of a non-decomposed sugar of nectar. Freshly harvested honey not yet sealed in the cells, i. e., not completely processed nectar, can have up to 6% sucrose. But in the sealed cells, the process of sucrose inversion continues, and therefore there is practically no sucrose left in the mature honey.
The composition of honey is still complex carbohydrates – dextrins – products of incomplete decomposition of starch. The total number of them is usually 1-4%, although in some cases their number may reach 12%. Dextrins are soluble in water and prevent the crystallization of honey. They are found in the stern that the bees have prepared from pure sugar, fed to them, which indicates the ability of bees to synthesize dextrin under the influence of enzymes in the anterior part of the digestive tract.
Protein substances (containing nitrogen) in honey are little, from 0,1 to 1,5% (on average 0,4-0,6%), but they all belong to water-soluble proteins and are easily absorbed in the intestine. Their origin is twofold: part comes from nectar and belongs to vegetable proteins, the other part comes along with the secretion of glands of the anterior part of the intestine and refers to animal proteins. In addition, there are also nitrogenous non-proteinaceous substances and some amino acids.
Honey contains acids (up to 0.43%), quite diverse in composition. Most of all organic acids, of which the main one is gluconic acid. In honey, found lactic, tartaric, oxalic, malic, citric, acetic, formic, as well as glutamic and aspartic acids (the latter are considered anti-crystallizers of sugars). From inorganic acids in honey contain phosphoric and hydrochloric.
The active acidity of honey is on average 3.78 (with variations from 3.26 to 4.36). Honey always has a clearly acidic reaction, which is important for the enzymatic processes taking place in honey. The taste of honey and its bactericidal properties depend on the value of active acidity.
Mineral substances of honey are very diverse (37 elements are found: many potassium, sodium, calcium, magnesium, iron, phosphorus), although they make up only 0.27% of dry matter. Of the main trace elements in 1 g of honey contains: 9.7 μg of iron, 4.2-manganese, 0.8-copper, 0.15 μg of cobalt. The amount of these substances varies greatly depending on the type of plants from which the nectar is harvested. It is interesting that the mineral composition of honey is very close to the mineral composition of human blood.
Aromatic substances. The aroma of plants from which nectar is collected is transferred to honey. In the composition of different honey, up to 120 substances affecting its aroma were found.
Colorants give honey this or that color: from golden amber to brown or dark.
There are few vitamins in honey, but they are in combination with other substances important for the body, and this increases their value. 1 g of honey contains 30 mg of ascorbic acid (C), 10-tocopherol (E), 4-pantothenic acid (B3), 3.8-biotin (H), 3.1-niacin, 3.0 μg pyridoxine (B2) and etc.
Honey is rich in enzymes. The most active of them are invertase, diastase, catalase. The role of invertase is already covered in the section on processing nectar in honey. Diastase decomposes starch. Its activity is determined by the diastase number, that is, by the number of milliliters of a 1% solution of starch decomposed per 1 hour by diastase contained in 1 g of honey.
The value of the diastatic number depends on many factors: the species composition of plants from which honey is prepared, the soil and climatic conditions, the weather, the intensity of nectar release, the strength of families,
The diastatic number of honey is often used as an indicator of its naturalness. At the Institute of Apiculture, diastatic numbers were determined for 80 samples of monoflair honey obtained from different regions of the country (Table 3).
Table 3. The diastatic numbers of some monoflour honey
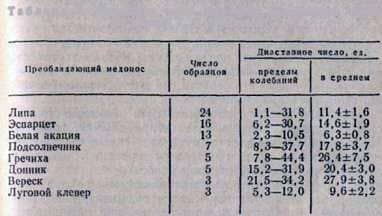
Thus, the diastatic number can only to a certain extent characterize the grade of honey. For an accurate determination of naturalness and good quality of honey, it is necessary to carry out additional studies, to determine the content of oxymethylfurfural, sucrose, reducing sugars, optical activity, aroma, taste, etc.
Less diastase activity is distinguished by honey collected by bees from spring honey-plants, more – from summer. The buckwheat honey is especially active in diastase.
After an annual storage, the activity of diastase decreases slightly. Catalase is an enzyme that decomposes hydrogen peroxide and plays a big role in the process of honey processing.
In small quantities, honey contains: protease, lipase, glycogenase, acid phosphatase, peroxidase, reductase, ascorbinoxidase, phospholipase, inulase, proteins, fats, and various intermediates formed in the cells of the body. This set of enzymes creates the conditions under which all honey substances can be decomposed and used in the cells of the body with the help of enzymes located right there in honey. All the components of honey, therefore, can be fully absorbed by the hibernating bee without any involvement of digestive enzymes. Such a high degree of preparation of honey for the assimilation and use of the cells of the body ensures the life of bees in winter, when the bee does not freeze at a low temperature, but the activity of its organs decreases drastically. This same feature of honey is one of its most valuable properties as a dietary and therapeutic product for humans.
Nectar has phytoncidal and bacteriostatic action. Phytoncids of nectar serve as one of the factors of natural immunity, which protects the reproductive organs of the flower from infection. They also impart antibiotic properties to honey.
The food that bees are made from sugar with top dressing does not contain all these substances, and therefore the sugar honey, although it resembles an externally natural bee, is very far from the chemical composition and the content of biologically active substances from natural honey.
When heating honey over 45 њ C part of the fructose forms oxymethylfurfural – a substance harmful to bees (but safe for humans). Therefore, if necessary, the crystallized honey should be dissolved. To do this, it is necessary to heat it only in a water bath and make sure that the water temperature does not exceed 50 њ C.
In assessing the quality of honey, it is important to keep water in it. Mature honey contains between 18 and 20% water. If the honey contains more water, it means that the processing of bees nectar into honey is not finished, it was pumped out on honey extractor from honeycombs with cells not yet sealed, i. e. not kept in the nest of bees until the end of processing. Immature honey is also characterized by an increased content of sucrose with a low content of glucose and fructose, a lower content of vitamins, enzymes, organic acids, aromatic substances, etc. It easily spoils due to spontaneous fermentation, its antimicrobial properties are less pronounced.
Toxic honey. In a number of cases (more often in the Caucasus mountains), bees collect nectar and pollen during the flowering of azalea, rhododendron, mountain laurel, andromeda, aconite, marshmallow marsh, common hare, chamois, etc. Nectar and pollen of these plants for bees are harmless, but have poisonous properties for a person. After consuming two to three tablespoons of honey, cold sweat, chills, vomiting, visual impairment, and even loss of consciousness appear. Usually the next day comes an improvement. No deaths were observed. Beekeepers of Georgia believe that such honey loses its virulence after six months of storage. Honey, freed from the seeds of rhododendron pollen, loses its toxic properties.
Chemical composition of honey
